5 Chapter 4: Fire Dynamics
Course Objectives
- Explain the basic principles of fire science. [4.3.11]
- Describe how thermal energy impacts fire behaviour. [4.3.11, 4.3.12]
- Explain the function of fuel within the combustion process. [4.3.10, 4.3.11]
- Explain the function of oxygen within the combustion process. [4.3.11]
- Explain the self-sustained chemical reaction involved in flaming combustion. [4.3.11]
- Differentiate among the stages of fire development. [4.3.11, 4.3.12]
- Explain how firefighting operations can influence fire behaviour in a structure. [4.3.11]
- Describe how building construction and layout affects fire development. [4.3.10, 4.3.11]
Fire dynamics describes the meeting point between fire science, materials science, fluid dynamics of gases, and heat transfer. Understanding the basic physics of these sciences can give firefighters the knowledge needed to forecast fire growth at a scene and predict the likely consequences of various tactical options available for controlling a fire.
Now, what?
Let’s get learning.
Lesson 1
Learning Objectives
- Explain the basic principles of fire science.
Fire Science
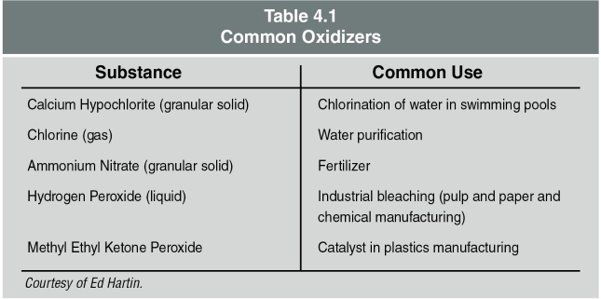
Firefighters should have a scientific understanding of combustion, fire, heat, and temperature. Fire can take various forms, but all fires involve a heat-producing chemical reaction between some type of fuel and an oxidizer, most commonly oxygen in the air. Oxidizers are not combustible but will support or enhance combustion. Table 4.1 lists some common oxidizers.
Physical Science Terminology

Physical science is the study of matter and energy and includes chemistry and physics. This theoretical foundation must be translated into a practical knowledge of fire dynamics. To remain safe, you need to be able to identify the fire dynamics present in any given situation and anticipate what the next stages of the fire will be along with how firefighting operations may impact the fire’s behaviour (Figure 4.1).
- The conditions found at a fire scene offer indications of a fire’s current behaviour and potential future behaviour. Courtesy of UL FSRI.
Identify fire dynamics - Anticipate the next stages of the fire
- Anticipate how operation may impact behaviour
The world around you is made up of matter in the form of physical materials that occupy space and have mass. While matter can undergo many types of physical and chemical changes, this chapter will concentrate on those changes related to fire.
Energy
The capacity to perform work. Work occurs when a force is applied to an object over a distance or when a substance undergoes a chemical, biological, or physical change. In the case of heat, work means increasing a substance’s temperature.

Forms of energy are classified as either potential or kinetic (Figure 4.3).
Potential energy represents the amount of energy that an object can release at some point in the future. Fuels have a certain amount of potential energy before they are ignited, based on their chemical composition.
The heat of combustion
The potential energy available for release in the combustion process. The rate at which a fuel releases energy over time depends on many variables including:
- Chemical composition
- Arrangement
- Density of the fuel
- Availability of oxygen for combustion
Kinetic energy
The energy that a moving object possesses. While a fuel such as wood is not “moving” as you might define it, when heat is introduced, the molecules within the fuel begin to vibrate. As the heat (thermal energy) increases, these molecules vibrate more and more rapidly. The fuel’s kinetic energy is the result of these vibrations in the molecules. There are many types of energy including:
- Chemical
- Thermal
- Mechanical
- Electrical
- Light
- Nuclear
- Sound
All energy can change from one type to another. For example, mechanical energy from a machine can convert to thermal energy when friction between moving parts generates heat. In terms of fire behaviour, the potential chemical energy of a fuel converts into heat and light during combustion.
Joules (J)
How energy is measured in the International System of Units (SI). The quantity of heat required to change the temperature of 1 gram of water by 1 degree Celsius is 4.2 joules.
British thermal unit (Btu)
In the customary system, this is the unit of measurement for heat. The amount of heat required to raise the temperature of 1 pound of water by 1 degree Fahrenheit. While not used in scientific and engineering texts, the Btu is still frequently used in the fire service. When comparing joules and Btu, 1 055 J = 1 Btu.
Chemical and physical changes almost always involve an exchange of energy. A fuel’s potential energy releases during combustion and converts to kinetic energy.
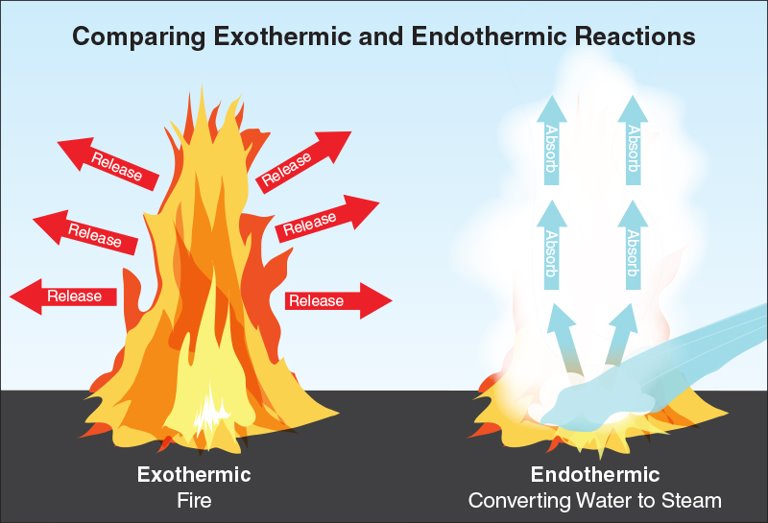
Exothermic reactions
Reactions that emit energy as they occur. Fire is an exothermic chemical reaction that releases energy in the form of heat and sometimes light.
Endothermic reactions
Reactions that absorb energy as they occur (Figure 4.4). For example, converting water from a liquid to a gas (steam) requires the input of energy resulting in an endothermic reaction. Converting water to steam is a tactic for controlling and extinguishing some types of fires.
Test Your Knowledge!
Fire Triangle and Tetrahedron
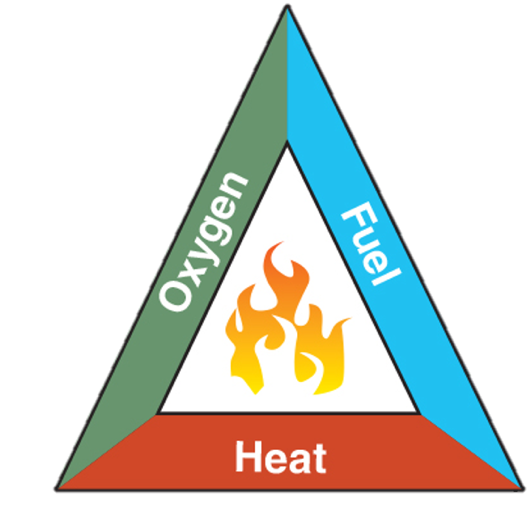
Fire triangle:
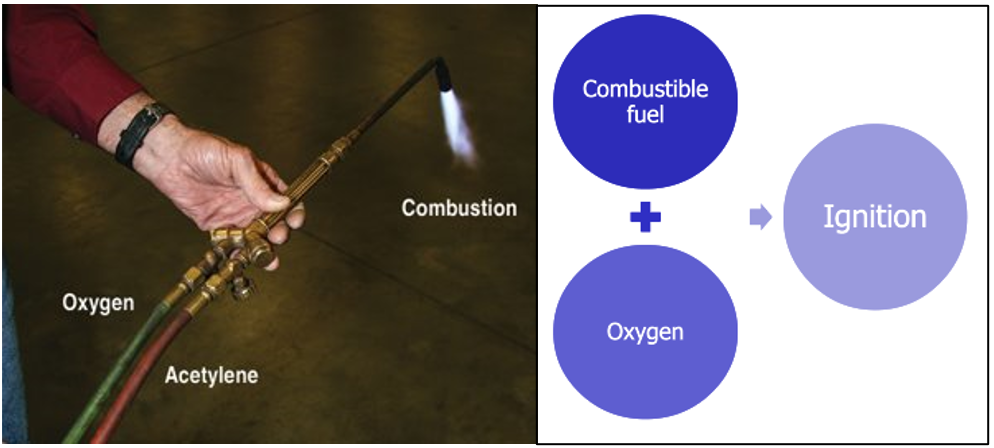
- The oldest and simplest model
- Shows three elements necessary for combustion to occur: fuel, oxygen, and heat. Remove any one of these elements and the fire will be extinguished.
- The fire triangle was used before the general adaptation of the fire tetrahedron, which includes a chemical chain reaction.
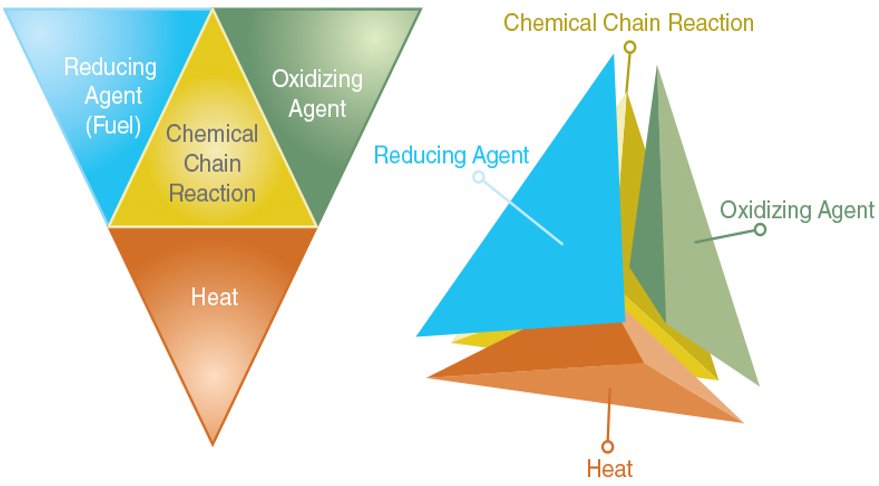
Fire Tetrahedron:
- Widely adopted today.
- An uninhibited chemical chain reaction must also be present for a fire to occur.
- Shows four elements necessary for combustion to occur: fuel (reducing agent), oxygen (oxidizing agent), heat and a chemical reaction. Remove any one of these elements and the fire will be extinguished.
- Includes the chemical chain reaction to explain flaming or gas-phase combustion (fire is an example of gas-phase combustion).
Ignition
Fuels must be in a gaseous state to burn; therefore, solids and liquids must become gaseous in order for ignition to occur. When heat is transferred to a liquid or solid, the substance’s temperature increases, and the substance starts to convert to a gaseous state (off-gassing).
Pyrolysis: Refers to the off-gassing chemical change in Solids.
Vaporization: Refers to the off-gassing chemical change in liquids. (Figure 4.7)
Piloted ignition: The most common form of ignition and occurs when a mixture of fuel and oxygen encounters an external heat source with sufficient heat or thermal energy to start the combustion reaction.

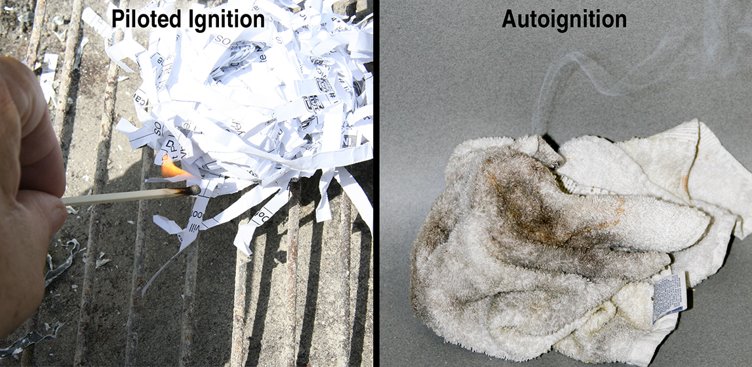
Autoignition: Occurs without any external flame or sparks to ignite the fuel gases or vapours. The fuel’s surface is heated to the point at which the combustion reaction occurs (Figure 4.8).
Once the fuel is ignited, the energy released from combustion transfers to the remaining solid fuel resulting in the production and ignition of additional fuel vapours or gases. This exchange of energy from the burning gases to the fuel results in a sustained combustion reaction.
Autoignition temperature (AIT): The minimum temperature at which a fuel in the air must be heated in order to start self-sustained combustion. The autoignition temperature of a substance is always higher than its piloted ignition temperature.
Combustion
Fire and combustion are similar conditions. Both words are commonly used to mean the same thing. Combustion, however, is a chemical reaction while flaming combustion is only one possible form of combustion. Combustion can occur without visible flames. There are two modes of combustion: non–flaming and flaming.
Non-flaming Combustion

Non–flaming Combustion
- Produces a smouldering glow at the material’s surface.
- Burning on or near the fuel’s surface at oxygen contact.
Non-flaming combustion occurs more slowly and at a lower temperature, producing a smouldering glow on the material’s surface. The burning may be localized on or near the fuel’s surface where it is in contact with oxygen. Examples of non-flaming or smouldering combustion include burning charcoal or smouldering wood or fabric. The fire triangle illustrates the elements/conditions required for this mode of combustion.
Flaming Combustion

Flaming Combustion
- The correct ratio of gaseous fuel mixes with oxygen and heats up to ignition temperature.
- Flames are produced above the material.
Flaming combustion is commonly referred to as fire. It produces a visible flame above the material’s surface. Flaming combustion occurs when a gaseous fuel mixes with oxygen in the correct ratio and heats to ignition temperature. Flaming combustion requires liquid or solid fuels to be converted to the gas phase through the addition of heat (vaporization or pyrolysis, respectively). When heated, both liquid and solid fuels will emit vapours that mix with oxygen, producing flames above the material’s surface if the gases ignite. The fire tetrahedron accurately reflects the conditions required for flaming combustion. Each element of the tetrahedron must be in the proper proportion and in close physical proximity for flaming combustion to occur. Removing any element of the tetrahedron interrupts the chemical chain reaction and stops flaming combustion. However, the fire may continue to smoulder depending on the characteristics of the fuel.
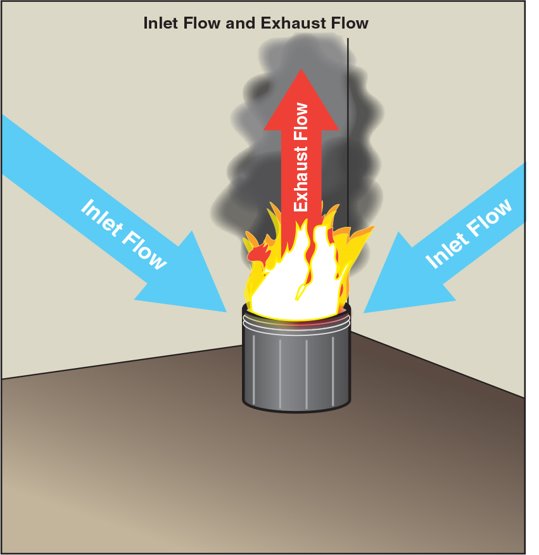
Air Entrainment
Ignition is where the combustion process begins. A heat source pyrolysis a fuel, creating fuel gases. Those gases mix with oxygen and ignite, creating a fire. The fire can be compared to a pump. Fresh oxygen is “pumped in” and mixes with fuel gases. Then as it burns, the fire “pumps out” combustion products that have larger amounts of mass and a higher level of energy than the inlet air. In the case of open burning, the “pump” does not have a well-defined inlet or outlet, as the air is being entrained (drawn in) from all around the burning fuel. Figure 4.9 illustrates the intake flow to the fire and the exhaust flow from the fire.
Products of Combustion
The fire generates heat. As the heat transfers to the gaseous combustion products, they expand and begin to rise and move away from the fire due to buoyancy. In other words, the density of the hot combustion products is less than the surrounding air, and the combustion products “float” on the dense cool air surrounding the fuel, creating the layers of smoke and fuel gases that fill a compartment during a fire.
As a fuel burns, its chemical composition changes, which produces new substances. These products of combustion are often simply described as heat and smoke. While the heat from a fire is a danger to anyone directly exposed to it, exposure to toxic gases found in smoke and/or lack of oxygen causes most fire deaths.
Smoke
An aerosol comprised of gases, vapour, and solid particulates.
- Smoke is the product of incomplete combustion.
- Combustion is incomplete when any of the fuel is left after combustion has occurred.
- Smoke and ash are examples of leftover fuel from incomplete combustion.

Figure 4. 10
By comparison, under ideal conditions, the entire fuel would undergo a chemical conversion from its current form into an equal amount of new materials. For example, the complete combustion of methane in the air results in the production of heat, light, water vapour, and carbon dioxide. However, combustion is incomplete in a structure fire, meaning that some of the fuel does not burn, but instead gets entrained with hot gases and rises aloft. This unburned fuel is smoke, and it has the potential to burn (Figure 4.10).
Most structure fires involve multiple types of fuels (carbon-based fuels [wood, cotton], hydrocarbon fuels
[plastics, synthetic fabrics], and other types), and the fires tend to have a limited air supply. When the air supply is limited, the level of incomplete combustion is higher, which produces more smoke. These factors result in complex chemical reactions that generate a wide range of products of combustion including toxic and flammable gases, vapours, and particulates that comprise smoke.
Structure Fire – Higher level of Incomplete Combustion
- Unburned fuel is smoke and has the potential to burn.
- Most involve multiple fuels.
- Tend to have a limited air supply.
- Incomplete combustion produces more smoke.

Gases such as carbon monoxide (CO) are generally colourless, while vapour and particulates give smoke its varied colours. Most components of smoke are toxic and dangerous to human life. The materials that makeup smoke vary from fuel to fuel, but generally all smoke is toxic. The toxic effects of smoke inhalation are the result of the interrelated effects of all the toxic products present.
Keep in mind that the combustion process consumes oxygen from the air, effectively removing it from the environment. As part of the chemical reaction, the consumed oxygen combines with carbon in the smoke to form combustion products like CO or carbon dioxide (CO2).
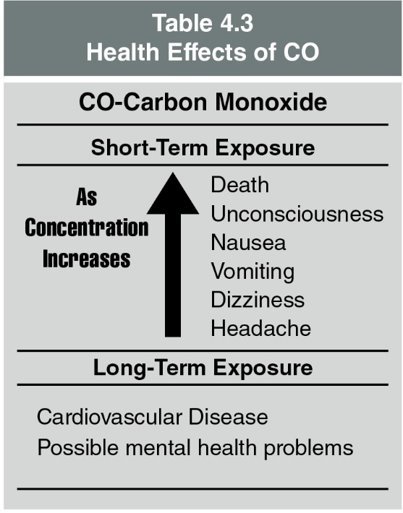
Low oxygen concentrations alone can result in hypoxia or death. The toxic gases in combination with a low oxygen concentration can reduce the time that a victim could survive. Table 4.2 lists some of the more common products of combustion and their toxic effects. Concentrations of the products of combustion and/or low oxygen concentrations can cause asphyxiation (a fatal level of oxygen deficiency in the blood).
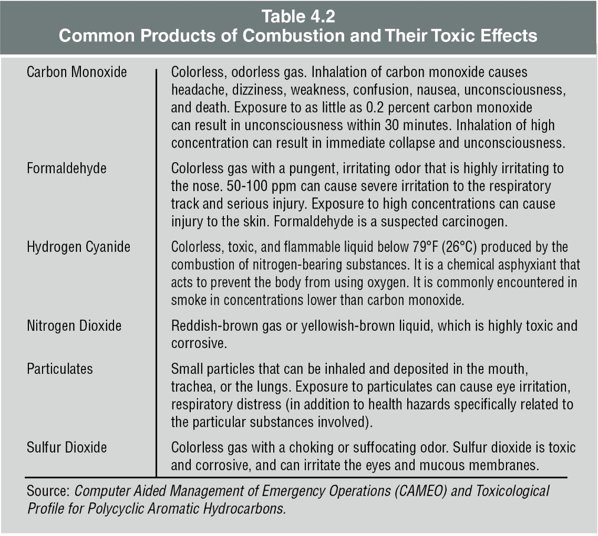
Carbon Monoxide (CO)
What is Carbon Monoxide (CO)?
- CO is an invisible, odorless gas resulting from incomplete burning of carbon-based materials.
- It is encountered at virtually all fires, indicating limited oxygen during combustion.
Sources of CO in Fires
- CO is produced by organic materials as they burn in environments with insufficient oxygen.
- Risks of CO:
- Recognized as a leading cause of death in fire-related civilian fatalities.
- Functions as a chemical asphyxiant, depriving organs of oxygen.
CO Poisoning
- A critical condition where CO binds with hemoglobin over 200 times more readily than oxygen.
- This binding reduces the blood’s oxygen-carrying capacity, causing brain and tissue hypoxia.
- Symptoms include headaches, nausea, dizziness, and unconsciousness, leading to death if unaddressed.
Note on CO Exposure
- Prolonged exposure to CO can cause long-term health issues such as cardiovascular disease and mental health problems.
- For a quick reference to the effects of CO on health, consult Table 4.3.
Hydrogen Cyanide (HCN)
What is Hydrogen Cyanide (HCN)?
- HCN is a dangerous by-product generated when nitrogen-containing materials burn.
- It is a toxic and flammable gas that can be inhaled, ingested, or absorbed through the skin.
Sources of HCN in Fires:
HCN is often produced during the incomplete burning of various materials, including:
- Natural fibers: Wool, cotton, silk
- Resins: Carbon fiber, fiberglass
- Synthetic polymers: Nylon, polyester
- Synthetic rubber: Neoprene, silicone, latex
These materials are commonly found in household and building items such as:
- Furnishings, bedding, insulation, carpets, clothing
Risks of HCN:
- HCN is present in smoke but typically at lower levels than carbon monoxide (CO).
- It is 35 times more toxic than CO, affecting the heart and brain by hindering oxygen use at the cellular level.
- HCN exposure can result from inhalation, ingestion, or skin absorption, leading to cell death.
- The severity of effects depends on the amount, concentration, and duration of exposure.
- High exposure can cause severe damage or be fatal.
Note on HCN Exposure:
- Vehicle fires and items with polyurethane foam are significant sources of HCN.
- Off-gassing during heating can also release HCN.
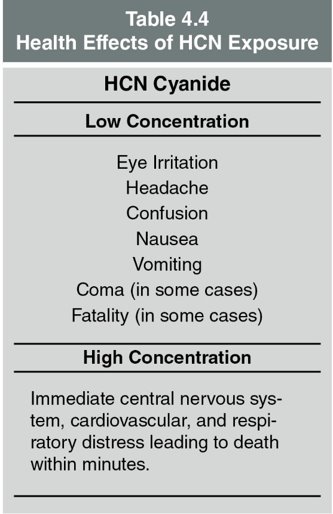
- Refer to Table 4.4 for detailed effects of HCN on the human body.

Carbon Dioxide (CO2)
What is Carbon Dioxide (CO2)?
- CO2 is a by-product of the complete combustion of organic materials.
- Unlike CO, CO2 is not toxic in the same manner but can create oxygen-deficient atmospheres.
Risks of CO2:
- CO2 can act as a respiratory stimulant, increasing the rate of breathing.
- At high concentrations, CO2 can cause central nervous system, cardiovascular, and respiratory distress, potentially leading to fatality within minutes.
Health Effects of CO2 Exposure
- Low concentrations can lead to eye irritation, headaches, confusion, nausea, and vomiting.
- In extreme cases, high CO2 levels can result in coma or fatality.
Immediate Risks at High Concentration
- Exposure to high concentrations of CO2 can cause immediate distress to the central nervous system, cardiovascular, and respiratory systems, leading to rapid health deterioration and death.
Note on CO2 Health effects
- Refer to Table 4.4 for a detailed understanding of the health effects of HCN exposure, which can accompany CO2 in fire situations.
- Fire gases contain a variety of other substances beyond CO2, CO, and HCN, each with their unique effects and critical exposure times.
- Irritants in smoke can cause discomfort and inflammation in the eyes and respiratory tract and can include substances like hydrogen chloride, formaldehyde, and acrolein.
- The presence of solid and liquid particulates, along with unburned fuels in smoke, can obstruct vision and impede breathing.
Fire gases contain many other gases than the three highlighted in this section. These additional gases have their own effects and exposure times. The exposure time is based on the combination of gases or the lethal effective dose.
Irritants in smoke are substances that cause breathing discomfort and inflammation of the eyes, respiratory tract, and skin. Smoke can contain a wide range of irritating substances depending on the fuels involved. More than 20 irritants in smoke have been identified including hydrogen chloride, formaldehyde, and acrolein.
Smoke also contains significant amounts of unburned fuels in the form of solid and liquid particulates and gases. Smoke must be treated with the same respect as any other flammable gas because it may burn or explode. Particulates can interfere with vision and breathing.

Pressure Differences
Atmospheric pressure (1 atmosphere [app. 101 kPa]) at standard temperature (68° F [20° C]) indicates the amount of pressure the atmosphere applies to the surface of the earth. At standard temperature and atmospheric pressure, gases remain calm and move very little. Differences in pressure above or below standard pressure create movement in gases. Gases always move from areas of higher pressure to areas of lower pressure. Even small differences in pressure, such as the 0.1 kPa or less differences created in most compartment fires, create this movement.
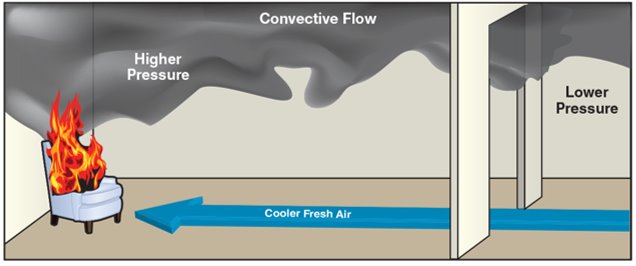
Implications for Firefighting
- The heat from a fire increases the pressure of surrounding gases, which then seek to balance expand and equalize with areas of lower pressure.
- This results in convective flow: heated gases rise – remain aloft (buoyant) – and up and out, simultaneously, cooler – fresher – air moves inward toward the fire.
- As the pressure difference between high- and low-pressure areas increases, the speed with which gases will move from high to low also increases.
- Understanding these subtle pressure changes is crucial for firefighters to predict fire behaviour and ensure safety (see Figure 4.11).
It is critical for firefighters to understand how small changes to the gas pressure within a structure can dramatically affect fire behaviour (Figure 4.11).
Test Your Knowledge!
Lesson 2
Outcomes:
- Describe how thermal energy impacts fire behaviour.
Thermal Energy (Heat)
A working knowledge of fire dynamics requires an understanding of temperature, energy, and power or heat release rate. Firefighters often use these terms interchangeably because the differences between the terms are not always understood.
Difference between Heat Release Rate and Temperature
As heat begins to vibrate the molecules in a fuel, the fuel begins a physical change from a solid or liquid to a gas. The fuel emits flammable vapours which can ignite and release thermal energy. This new source of thermal energy begins to heat other, uninvolved fuels converting their energy and spreading the fire.
A block of wood at room temperature has stable molecules and is in no danger of ignition. When thermal energy transfers to the wood, the wood is heated, and the temperature of the wood rises because the molecules have begun to vibrate and move more freely and rapidly.
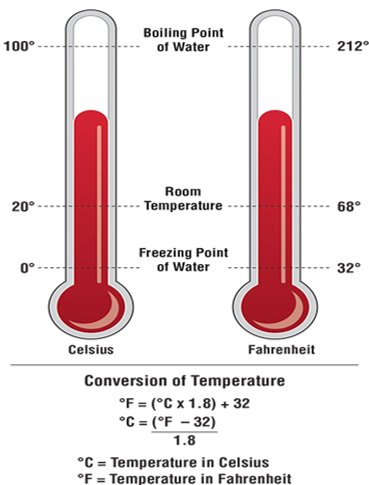
Temperature can be measured using several different scales. The most common are the Celsius scale, used in the International System of Units (SI) (metric system), and the Fahrenheit scale, used in the Imperial system. The freezing and boiling points of water provide a simple way to compare these two scales (Figure 4.12).
- Measurement of heat is measured in Celsius (SI units) or Fahrenheit (Imperial units), with water’s freezing and boiling points as a reference.
- Not an accurate measurement of heat transfer
- On its own, it cannot accurately indicate the danger in a fire scenario
Heat Release Rate
A dangerous misconception is that temperature is an accurate predictor or measurement of heat transfer. It is not. For example, one candle burns at the same temperature as ten candles. However, the heat release rate (kW) of the ten candles is ten times greater than one candle at the same temperature. The increased heat release rate results in an increased heat transfer rate to an object. This energy flow to a unit area (heat flux) is measured in kilowatts per square meter.
Translated to an interior fire environment, the temperature in the structure may be within tolerances for personal protective equipment however, the heat flux to the PPE from the fire indicates the real measurement of how long the PPE will protect you.
** NOTE: The temperature tells you it is safe to go in, but the heat transfer rate – not the temperature – tells you how long you can stay in. **
Sources of Thermal Energy
Chemical, electrical, and mechanical energy are common sources of heat that result in the ignition of a fuel. They can all transfer heat, cause the temperature of a substance to increase, and are most frequently the ignition sources of structure fires.
Chemical Energy
Chemical energy is the most common source of heat in combustion reactions. The potential for oxidation exists when any combustible fuel is in contact with oxygen. The oxidation process almost always results in the production of thermal energy (Figure 4.13).
Spontaneous ignition: Ignition without the addition of external heat. Self-heating can lead to this.
Oxidation and Spontaneous Ignition
Understanding Oxidation
- Oxidation normally produces thermal energy slowly. The energy dissipates almost as fast as it is generated.
- External heat sources, like sunlight, can start or speed up this process.
Conditions for Self-heating Spontaneous Ignition
- For a substance to self-ignite, it must retain heat faster than it’s lost due to the insulation properties of surrounding materials.
- The heat production rate must be sufficient to bring the material to its autoignition temperature.
- There must be enough oxygen available to sustain combustion.
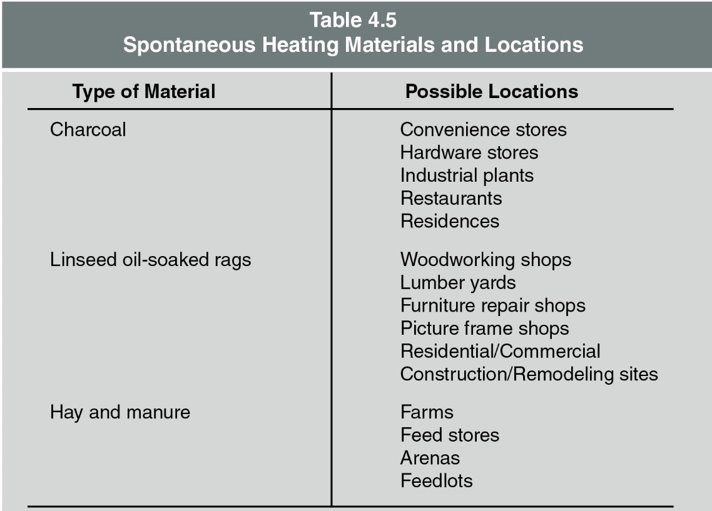
Example of Spontaneous Ignition
- Linseed oil-soaked rags balled up and left in a corner can spontaneously ignite.
- The heat generated by the oxidation of the oil is trapped by the cloth, which can lead to an increase in temperature and eventually cause ignition.
Chemical Reaction Rates and Temperature
- The rate of chemical reactions, including oxidation, generally increases with the temperature of the materials involved.
- If the generated heat exceeds the dissipated heat, the material may reach its autoignition temperature and ignite spontaneously.
Electrical Energy
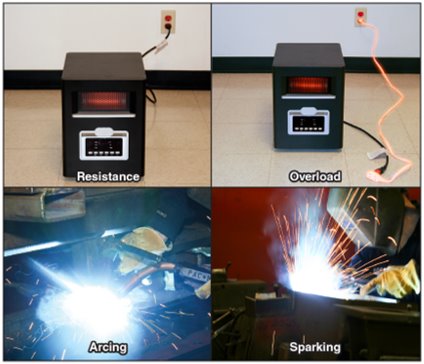
Electrical energy can generate temperatures high enough to ignite any combustible materials near the heated area.
Examples of Electrical Heating (Figure 4.14)
- Resistance Heating
Heat produced by electric current flowing through a conductor. Devices like incandescent lamps, ranges, ovens, or portable heaters, are designed to make use of resistance heating. Other electrical equipment is designed to limit resistance heating under normal operating conditions. - Overcurrent/Overload
When the current flowing through a conductor exceeds its design limits, the conductor may overheat and present an ignition hazard. Overcurrent or overload is unintended resistance heating. - Arcing
A high-temperature luminous electric discharge across a gap or through a medium (i.e. charred insulation). often generated when there is a gap in a conductor such as a cut or frayed wires, or when there is high voltage, static electricity, or lightning. - Sparking
Occurs alongside arcing, where luminous (glowing) particles may splatter and ignite nearby materials.
Mechanical Energy
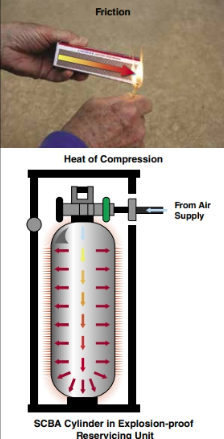
Friction and compression generate mechanical energy (Figure 4.15). The movement of two surfaces against each other creates heat of friction that generates heat and/or sparks. Heat is generated when a gas is compressed.
Friction:The movement of surfaces against each other increases temperature, potentially causing ignition.
Compression: The process of applying a force to a material or object, causing it to decrease in volume and become more compact.
E.g. Diesel engines use this principle to ignite fuel vapours without spark plugs. This principle is also the reason that SCBA cylinders feel warm to the touch after they are filled. When a compressed gas expands, the gas absorbs heat. This absorption accounts for the way the cylinder cools when a CO2 extinguisher is discharged.
Heat Transfer
 Heat transfer is a key concept in thermodynamics, which is the science of how heat moves and behaves. In fire dynamics, heat transfer from the initial fuel package (burning object) to other fuels in and beyond the fire’s origin, affects the growth of every fire.
Heat transfer is a key concept in thermodynamics, which is the science of how heat moves and behaves. In fire dynamics, heat transfer from the initial fuel package (burning object) to other fuels in and beyond the fire’s origin, affects the growth of every fire.
Heat Transfer and Fire Growth
- When something burns, the heat from this fuel package doesn’t stay put; it moves outwards to nearby objects. This movement of heat can cause those objects to catch fire too, leading to the growth of the fire.
Thermal Equilibrium
- State in which all areas of an object have a uniform temperature.
- Heat has a natural tendency to spread out evenly. This means it will always move from a hotter area (like a burning log) to a cooler area (like a nearby curtain) until both are at the same temperature. When two objects reach the same temperature, heat transfer between them stops because they’ve achieved what’s known as thermal equilibrium.
Rate of Heat Transfer
- The rate at which heat transfers is related to the temperature differential of the bodies and the thermal conductivity of the materials involved.
- Temperature Differential: The speed at which heat moves from one object to another depends on the temperature difference between them. If one body is much hotter than the other, heat will transfer more quickly.
- Thermal Conductivity: This is a property of a material that indicates how well it can transfer heat. Materials with high thermal conductivity, like metals, transfer heat faster than those with lower conductivity, like wood or glass.
Heat can be transferred through three mechanisms: Conduction, Convection, and Radiation.
- Conduction
This is the process where heat moves through a material or between materials that are in direct contact. It’s like when a metal spoon gets hot after being left in a pot of boiling water. - Convection
This occurs when heat is carried away by a fluid, such as air or water. For instance, when hot air rises from a radiator and circulates around a room. - Radiation
Heat can also be transferred through electromagnetic waves, similar to how the sun heats the Earth. An example is feeling the warmth of a fireplace even when you’re not directly next to it.
Conduction
Conduction occurs when heat is transferred between solid objects, in this case, between the door and the firefighter’s hand.
Conduction is the transfer of heat through and between solids. It occurs when a material is heated as a result of direct contact with a heat source:
- Heat Source Contact
Conduction occurs when a material is heated as a result of direct contact with a heat source (Figure 4.16). - Molecular Activity
Conduction results from increased molecular motion and collisions between a substance’s molecules, resulting in the transfer of energy through the substance. - Efficiency of Conduction
The more closely packed the molecules of a substance are, the more readily it will conduct heat. - E.g. If a fire heats a metal pipe on one side of a wall, the heat conducted through the pipe can ignite wooden framing components in the wall or nearby combustibles on the other side of the wall.
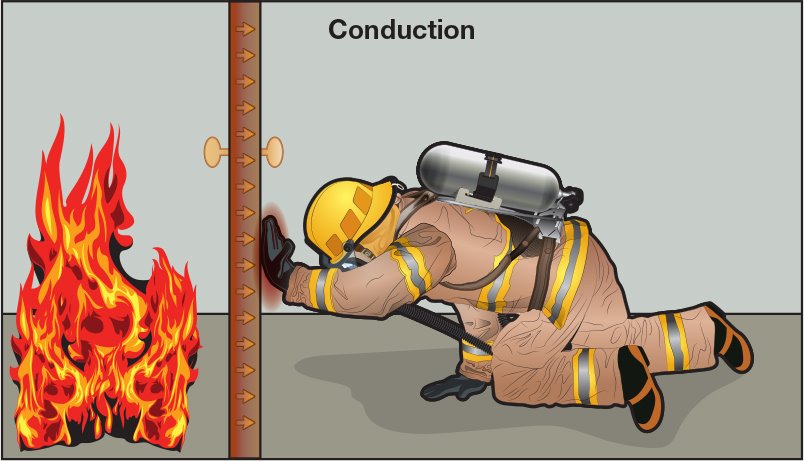
Figure 4. 16 Conduction occurs when heat is transferred between solid objects, in this case, between the door and the firefighter’s hand.
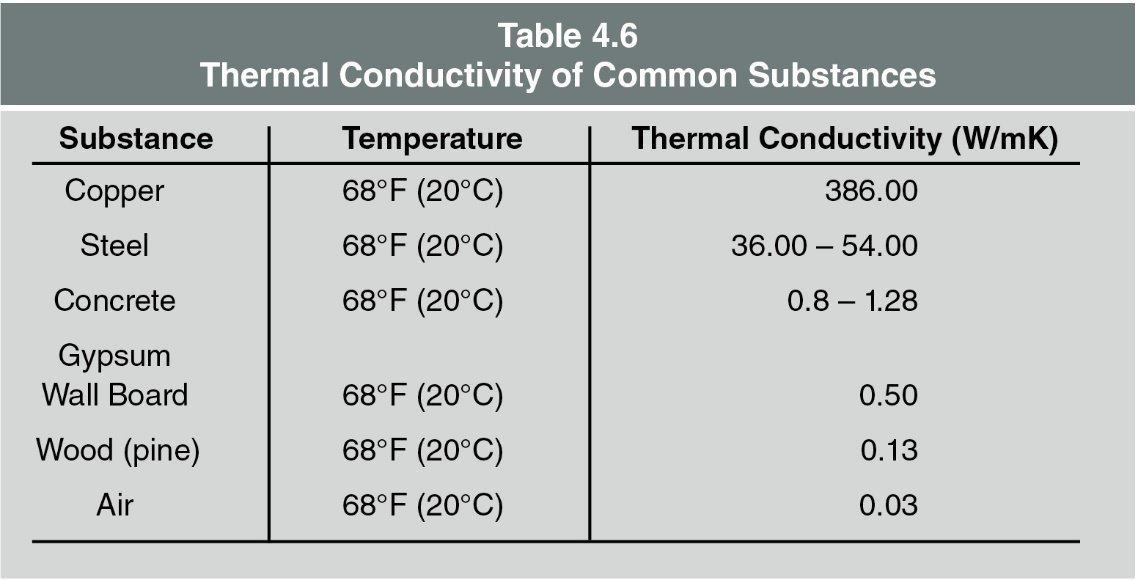
Heat transfer due to conduction is dependent upon three factors:
- Area Being Heated
The size of the area being heated affects how much heat is transferred. A larger area means more space for heat to flow into. - Temperature Gradient
The difference in temperature between the heat source and the material being heated drives the process. The larger the difference, the faster the heat transfer. - Material Conductivity
This is about how well a material allows heat to pass through it. Each material has its own rate, which can be seen in Table 4.6. For instance, copper conducts heat much faster than steel, and steel conducts heat much faster than concrete.- Insulating Materials
Materials that slow the conduction of heat. A good insulator’s structure – often made of fine particles or fibres with void spaces between them filled with a gas such as air – disrupts the point-to-point transfer of heat or thermal energy. Since air and gas conduct heat poorly due to the wide spacing of their molecules, they’re excellent insulators.
- Insulating Materials
Convection
Convection is the transfer of thermal energy by the circulation or movement of a fluid (liquid or gas) (Figure 4.17). In the fire environment, convection usually involves the transfer of heat through the movement of hot smoke and fire gases:
- Heat Transfer
As with all heat transfer, the heat flows from the hot fire gases to the cooler structural surfaces, building contents, and air. - Movement
Convection may occur in any direction. Vertical movement is due to the buoyancy of smoke and fire gases. Lateral movement is usually the result of pressure differences (movement from high to low pressure).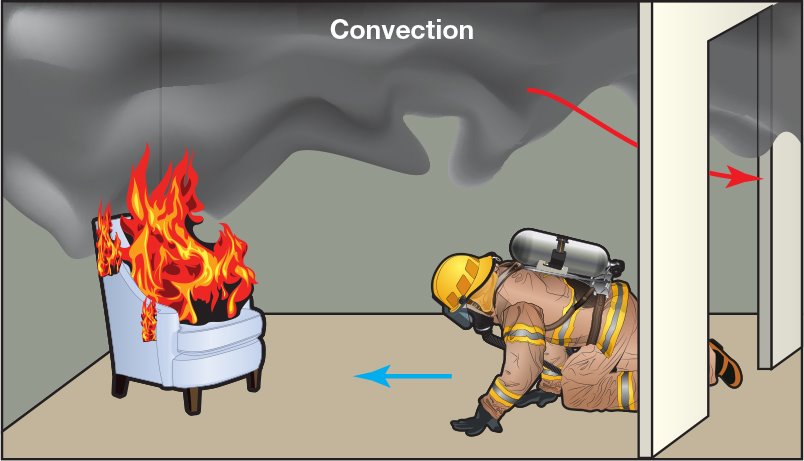
Figure 4. 17 Convection is the transfer of heat by the circulation of liquids or gases
Convection is dependent upon three factors:
- Area Being Heated
The extent of the area exposed to hot gases impacts how much heat is transferred. - Temperature Gradient
The greater the difference in temperature between the hot gases and the cooler materials, the more intense the heat transfer. - Gases’ Behavior
The turbulence and velocity of moving gases. The more turbulent or faster-moving the gases, the more effectively they can transfer heat.
Radiation
Radiation is the transmission of energy as electromagnetic waves, such as light waves, radio waves, or X-rays, without an intervening medium (Figure 4.18). Radiant heat can become the dominant mode of heat transfer as the fire grows in size and can have a significant effect on the ignition of objects located some distance from the fire. Radiant heat transfer is also a significant factor in fire development and spread in compartments.
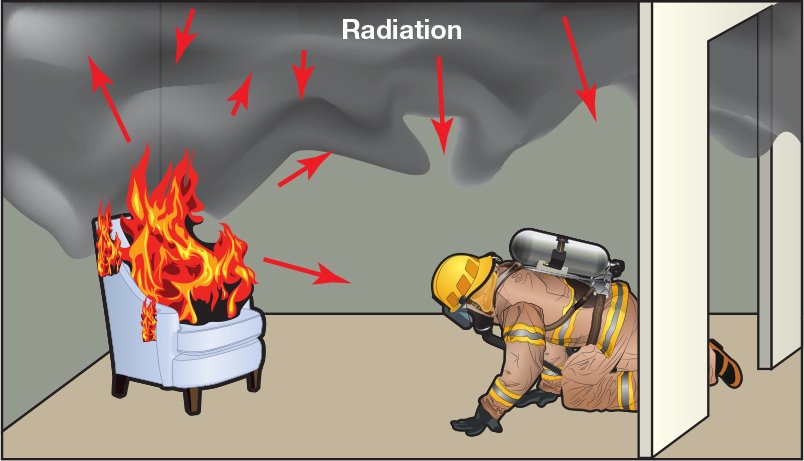
Radiation
- Transmission of energy as electromagnetic waves
- Can become dominant mode of heat transfer as fire grows
- Can significantly affect objects located a distance from the fire
- Significant factor in compartment fire development and spread
Influencing Factors of Radiant Heat Transfer
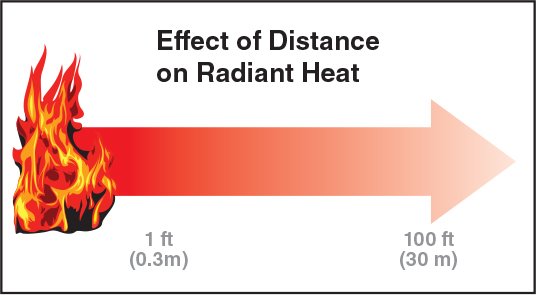
Nature of the exposed surfaces
Dark-coloured materials emit and absorb heat more effectively than light-coloured materials; smooth or highly-polished surfaces reflect more radiant heat than rough surfaces.
Distance: The distance between the heat source and the exposed surfaces. Increasing distance reduces the effect of radiant heat (Figure 4.19).
Temperature of the heat source
Unlike other methods of heat transfer that depend on the temperature of both the heat source and exposed surface, radiant heat transfer primarily depends on the temperature of the heat source. As the temperature and heat release rate of the heat source increases, the radiant energy also increases (Figure 4.20)

Properties of Radiant Heat
- As an electromagnetic wave, radiated heat energy travels in a straight line at the speed of light.
- E.g. The heat of the sun. The energy travels at the speed of light from the sun through space (a vacuum) until it strikes and warms the surface of the earth.
- Radiant heat from a fire can ignite objects far from the actual flames by travelling through air spaces, which would otherwise impede conduction and convection.
Radiation and Fire Growth
- Radiation is a common cause of exposure fires: a fire resulting from another fire outside that building, structure, or vehicle, or a fire that extends to an outside property from a building, structure, or vehicle.
- As a fire grows, it radiates more energy which other objects absorb as heat. In large fires, the radiated heat can ignite buildings or other fuel packages a considerable distance away.
- Radiated heat travels through vacuums and air spaces that would normally disrupt conduction or convection.
- Materials that reflect, absorb, or scatter radiated energy will disrupt the heat transmission.
- While flames emit considerable radiant energy, so can hot smoke and fire gases in the upper layer of a fire environment.
Interaction Among the Methods of Heat Transfer
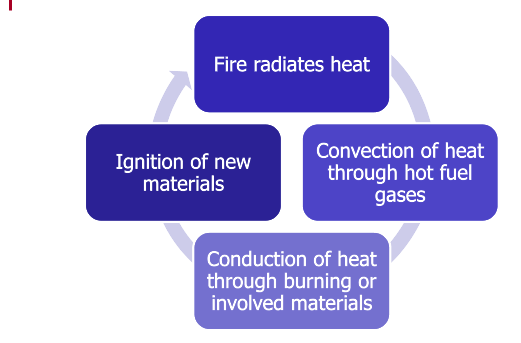 The methods of heat transfer rarely occur individually during a fire. The fire radiates heat, causes convection of heat through hot fuel gases, and conducts heat through burning materials or metals that are involved in the fire.
The methods of heat transfer rarely occur individually during a fire. The fire radiates heat, causes convection of heat through hot fuel gases, and conducts heat through burning materials or metals that are involved in the fire.
Convected heat and radiated heat that reaches walls and ceilings heat those surfaces which, in turn, begin to conduct heat to whatever extent possible based on the material’s thermal conductivity (Table 4.6). One side of the object is warm and slowly warms through the object until the opposite side is of equal temperature with the heated side. A heated surface will then, in turn, begin to radiate heat which could lead to ignition, combustion, convection, and so on. This cycle continues until interrupted.
The longer a firefighter remains in a heated environment, the more their PPE will absorb heat, gradually conducting it inward due to its low thermal conductivity until the interior matches the exterior temperature.
An example of this interaction is how your PPE absorbs heat during interior operations. Convected and radiated heat will begin to heat the exterior of your PPE. The longer you are in the heated environment, the more heat that surface will absorb. The PPE has low thermal conductivity, so it will conduct heat slowly. However, eventually, the interior surface of the PPE will heat to the same level as the exterior. Wherever the gear is compressed against skin or underclothing, heat will be conducted faster. Table 4.7 shows various responses of human skin and PPE as they are heated.
Where the PPE is not in contact, it will radiate heat to the insulating air layer between your body and the interior surface of the gear. This transferred heat can cause heat stress and will eventually cause PPE to fail. The heat absorption and build-up in PPE is a direct result of all of the heat transfer methods acting at the same time.
Test Your Knowledge!
Lesson 3
Outcomes:
- Explain the function of fuel within the combustion process.
Fuel
Fuel is the oxidized or burned material or substance in the combustion process. A fuel may be found in any of three physical states of matter: gas, liquid, or solid. The fuel in a combustion reaction is known as the reducing agent. Fuels may be inorganic or organic.
They can be divided into hydrocarbon-based fuels:
- Gasoline
- Fuel oil
- Plastics
- Cellulose-based materials (wood and paper)
A fuel’s Chemical Content
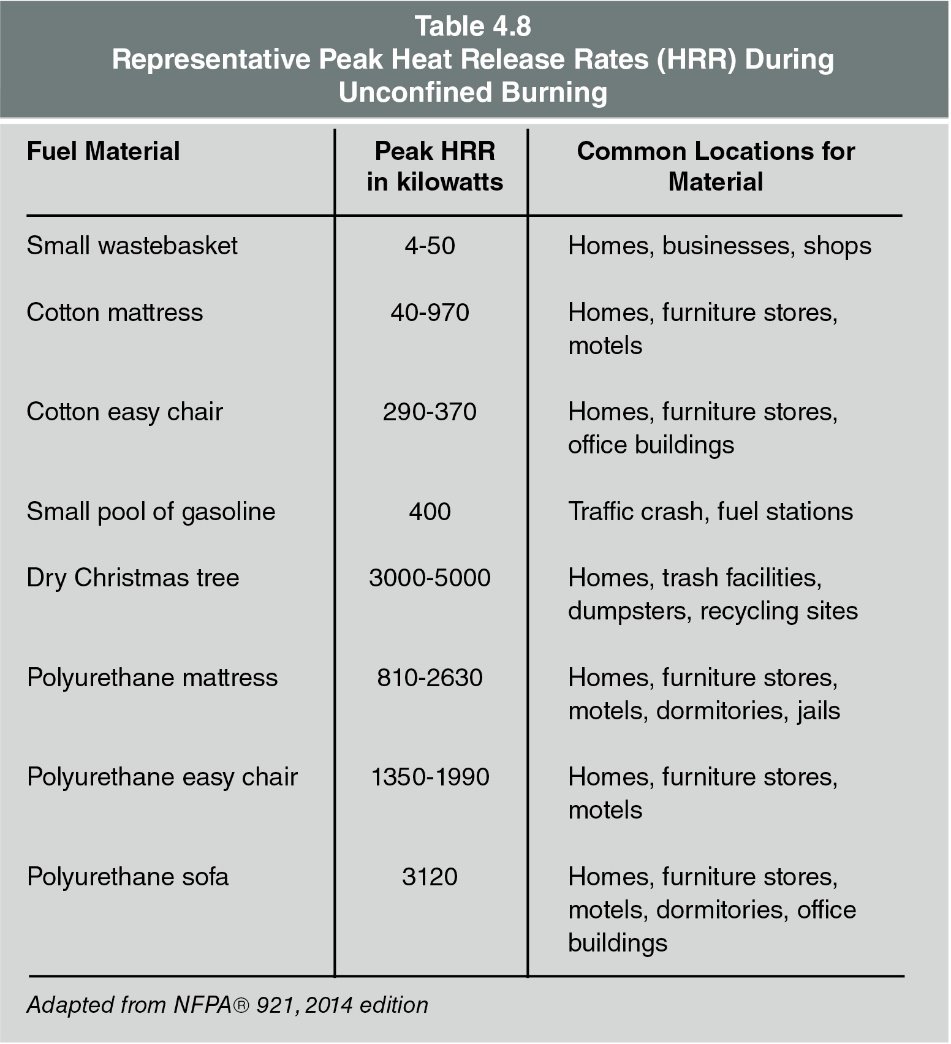 A fuel’s chemical content influences both its heat of combustion and heat release rate (Table 4.8).
A fuel’s chemical content influences both its heat of combustion and heat release rate (Table 4.8).
Fuel’s Heat of Combustion
The fuel’s total amount of thermal energy released when a specific amount of that fuel burns. In other words, different materials release more or less heat than others based on their chemical makeup. E.g. Many plastics, flammable liquids, and flammable gases contain more potential thermal energy than wood.
Synthetic Materials
These are common in modern construction and furnishings. These materials are synthesized from petroleum products, and as a result, they have higher heats of combustion and may generate higher heat release rates than wood on a per-mass basis.
Power: The rate at which energy transfers.
- Another way to describe power is the rate at which energy converts from one form to another. The standard international (SI) unit for power is the watt (W). One watt is 1 joule per second (J/s).
-
In fire behaviour, power is the heat release rate (HRR) during combustion. When a fuel is heated, work is being performed (energy is being transferred).
- The speed it is transferred, HRR, is the amount of generated power.
- It depends on the type, quantity, and orientation of the fuel.
- Examples of heat release rate conditions that may be measured in watts, kilowatts, or megawatts
HRR directly relates to oxygen consumption, as the combustion process requires a continuous supply of oxygen to continue.
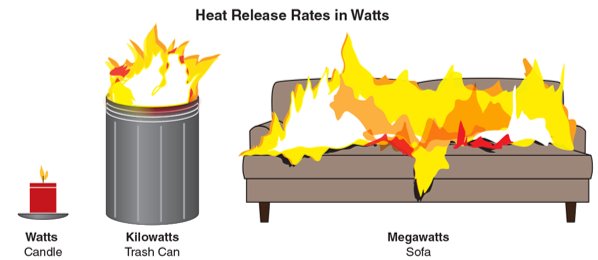
Typically, the more oxygen is available, the higher the HRR. Similarly, the HRR decreases if all available oxygen is consumed and not replenished. Figure 4.21 shows an example of the heat release rate produced by various sizes of fires.
Gases
For flaming combustion to occur, fuels must be in the gaseous state. As previously described, thermal energy is required to change solids and liquids into the gaseous state.
Vapour: The common term used to describe the gaseous state of a fuel that would normally exist as a liquid or a solid at standard temperature and pressure.
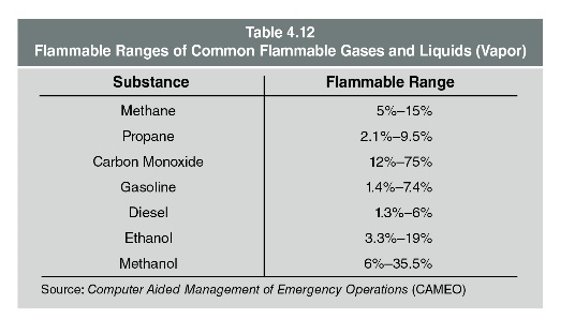
Table 4.9 contains characteristics of common flammable gases.
- Gaseous fuels such as methane (natural gas), hydrogen, and acetylene, can be the most dangerous of all fuel types because they are already in the physical state required for ignition.
- When wood burns inefficiently, the combustion products may contain methane, acetylene and other fuel gases.
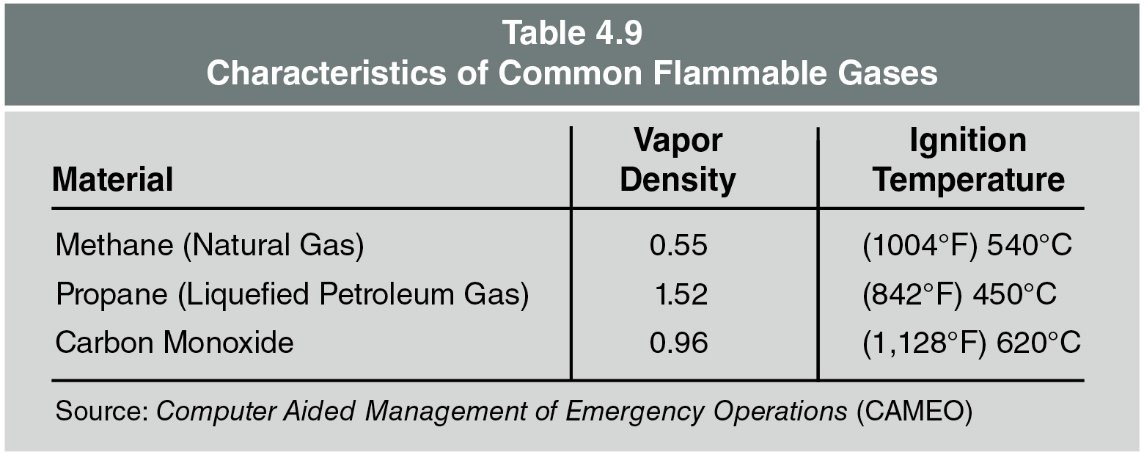
Vapour density: Describes the density of gases in relation to air.
- Air has a vapour density of 1. Gases with a vapour density of less than 1, such as methane, will rise while those having a vapour density of greater than 1, such as propane, will sink (Figure 4.22).
- Vapour densities are based upon the assumption that the density is measured at standard temperature and pressure.
- The vapor density of a gas provides an indication of where a gas will collect at an incident.
- Heated gases expand and become less dense; when cooled they contract and become more dense.
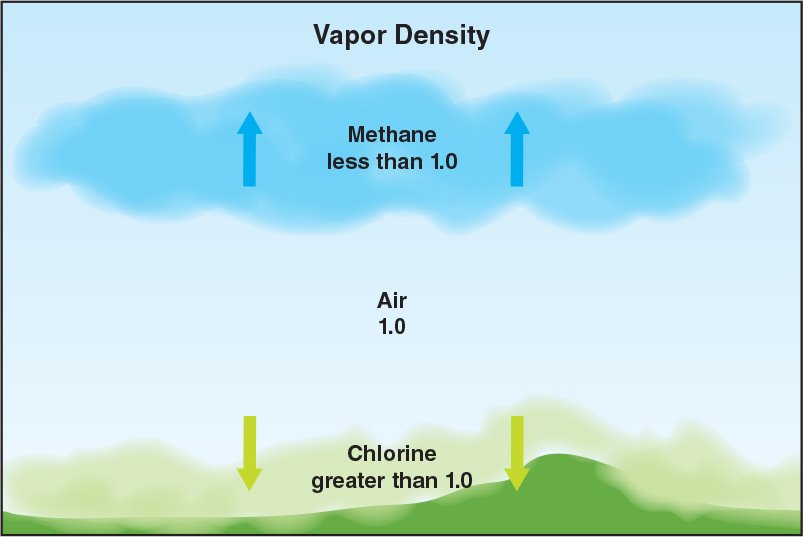
Figure 4. 22 The vapour density of a gas provides an indication of where a gas will collect at an incident.
Liquids
 Liquids have mass and volume but no definite shape except for a flat surface or the shape of their container. Unlike gases, liquids will not expand to fill all of a container. When released on the ground, liquids will flow downhill and pool in low areas.
Liquids have mass and volume but no definite shape except for a flat surface or the shape of their container. Unlike gases, liquids will not expand to fill all of a container. When released on the ground, liquids will flow downhill and pool in low areas.
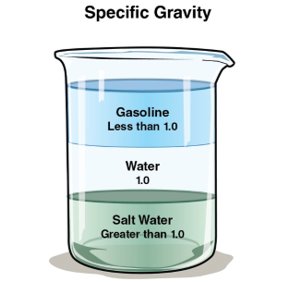
- Liquid density is compared to water. Water is assigned a specific gravity of 1. Liquids with a specific gravity less than 1, such as gasoline and most other flammable liquids, are lighter than water and will float on its surface. Liquids with a specific gravity greater than 1, such as corn syrup, are heavier than water and will sink (Figure 4.23).
Specific Gravity: The ratio of the mass of a given volume of a liquid compared to the mass of an equal volume of water at the same temperature.
Vapours
The specific gravity of a liquid indicates whether the liquid will float on the surface of water or sink.
- Vaporization: The transformation of a liquid to vapour or a gaseous state. Unlike solids, liquids retain their state of matter partly due to standard atmospheric pressure. For vaporization to occur, the escaping vapours must be at a greater pressure than atmospheric pressure.
- Vapour pressure: The pressure that vapours escaping from a liquid exerts. Vapour pressure indicates how easily a substance will evaporate. Flammable liquids with a high vapour pressure present a special hazard to firefighters.
- Rate of Vaporization: The vapour pressure of the substance and the amount of thermal energy applied to it determines the rate of vaporization.
- E.g. A puddle of water eventually evaporates because of the slow heat transfer from the sun. When the same amount of water is heated on a stove, however, it vaporizes much more rapidly because there is more thermal energy applied.
- Volatility of a Liquid: The ease with which a liquid gives off vapour influences how easily it can ig
 nite. The size of a liquid’s surface area also influences the extent to which the liquid will give off vapour. In many open containers, the surface area of liquid exposed to the atmosphere is limited.
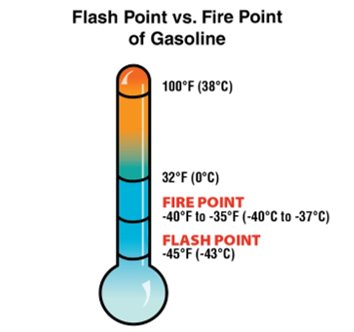
nite. The size of a liquid’s surface area also influences the extent to which the liquid will give off vapour. In many open containers, the surface area of liquid exposed to the atmosphere is limited. - Flash Point: The minimum temperature at which a liquid gives off sufficient vapours to ignite, but not sustain combustion, in the presence of a piloted ignition source.
- Commonly used to indicate the flammability hazard of liquid fuels. Liquid fuels that vaporize sufficiently to burn at temperatures under 100°F (38°C) present a significant flammability hazard.
- Fire Point: The temperature at which a piloted ignition of sufficient vapours will begin a sustained combustion reaction (Figure 4.24).
Solubility
Firefighters must know how liquid fuels react with water. Solubility describes t
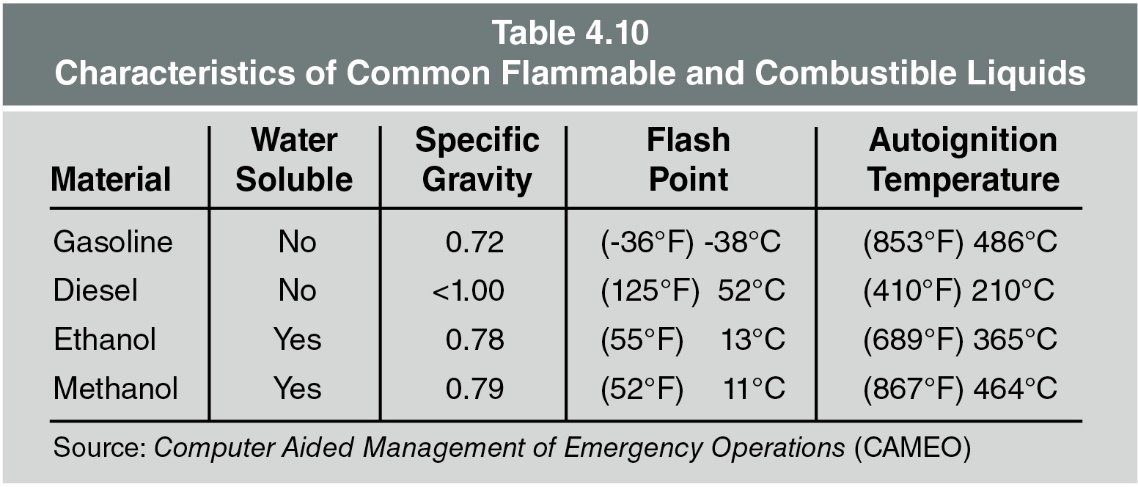
he extent to which a substance (in this case a liquid) will mix with water. Solubility may be expressed in qualitative terms (slightly or completely) or as a percentage (20 % soluble). Some liquids are lighter than water and do not mix with it, such as hydrocarbon fuels (gasoline, diesel, and fuel oil). Table 4.10 lists the characteristics of common flammable and combustible liquids.
Because the liquid fuel is less dense and will not mix with water, adding water to the liquid fuel may disperse the burning liquid instead of extinguishing it, which could potentially spread the fire to other areas.
Firefighters should use the appropriate foam or chemical agent to extinguish liquid fuels that are not water-soluble.
Miscible Materials: Materials that when in water will mix in any proportion.
Polar Solvents: Flammable liquids such as alcohols (methanol, ethanol) that will mix readily with water.
Water-soluble liquids: These liquids will mix with some water-based extinguishing agents, such as many types of fire-fighting foam.
- The extinguishing agent will mix with the burning liquid and become much less effective at extinguishing the fire. To avoid this mixture, firefighters should use alcohol-resistant fire-fighting foams specifically designed for polar solvents.
Solids
Solids have definite size and shape. Different solids react differently when exposed to heat. Some solids such as wax and metals will change their state and melt, while others such as wood and plastics will not. When solid fuels are heated, they begin to pyrolyze (off-gas) and release fuel gases and vapours. The solid fuels begin to decompose and emit combustible vapours. If there is enough fuel and heat, the process of pyrolysis generates sufficient flammable vapours to ignite in the presence of sufficient oxygen or another oxidizer.
Pyrolysis
Pyrolysis is the chemical decomposition of a material through heat. In firefighting, this refers to the process where materials break down and release flammable gases that can fuel a fire before actual ignition occurs.
When wood first heats, it begins to pyrolyze and decompose into its volatile components and carbon, often producing white vapours. Pyrolysis of wood begins at temperatures below 400°F (204°C) and is lower than the temperature required for the ignition of its vapours. Modern home construction uses wood for structural components and a variety of synthetic materials (like polyvinyl chloride, polyethylene, polystyrene, polypropylene, and polyurethane) for insulation and finishes. This includes common household items made of flexible polyurethane foam and differs in pyrolysis from wood as indicated in Table 4.11.
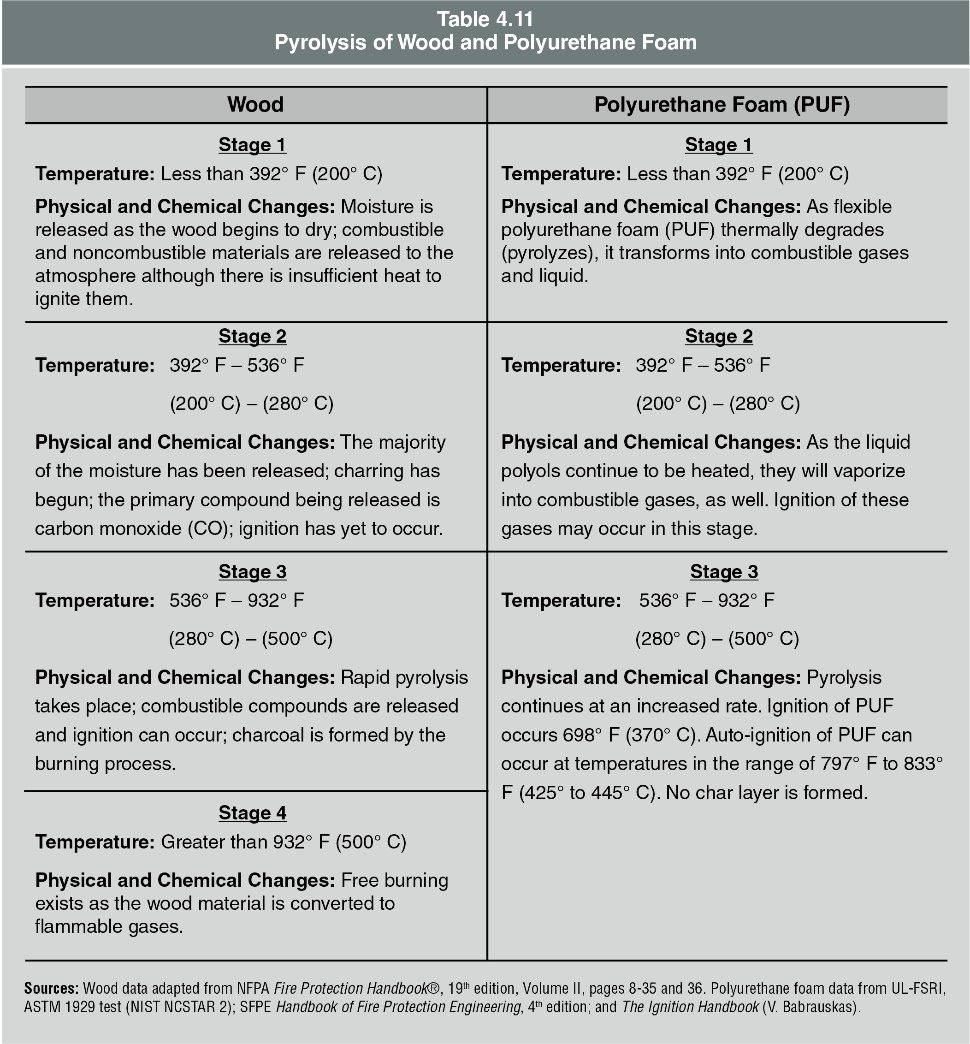
Solid Fuel Ignition

Solid fuels have a definite shape and size which significantly affects how easily they ignite. The primary consideration is the Surface-to-mass ratio: The surface area of the fuel in proportion to its mass.
For example, let’s take a look at a large tree:
- To produce lumber, the tree must be felled and cut into a log. The surface area of this log is low compared to its mass; therefore, the surface-to-mass ratio is low.
- The log is then cut into planks. This reduces the mass of the individual planks compared to the log. The resulting surface area increases, thus increasing the surface-to-mass ratio.
- The chips and sawdust produced as the planks are cut into boards have an even higher surface-to-mass ratio.
- If the boards are milled or sanded, the shavings or sawdust have the highest surface-to-mass ratio of any of the examples.
As this ratio increases, the fuel particles become more finely divided, like shavings or sawdust. Therefore, the particles’ ability to ignite increases exponentially. As the surface area increases, more of the material is exposed to the heat and generates combustible pyrolysis products more quickly (Figure 4.25).
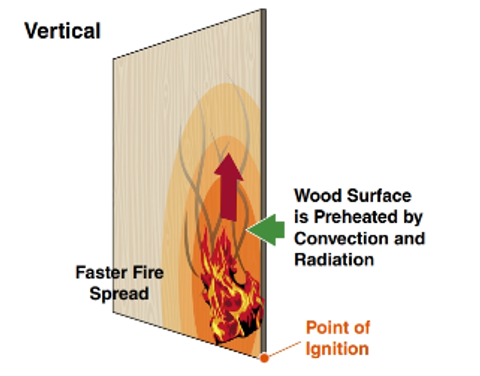
The proximity and orientation of a solid fuel relative to the source of heat also affect the way the fuel burns (Figure 4.26).
For example, if you ignite one corner of a sheet of 1⁄8-inch (3 mm) plywood panelling that is lying horizontally (flat), the fire will consume the fuel at a relatively slow rate. The same type of panelling in a vertical position (standing on edge) burns much more rapidly because the heated vapours rise over more surface area and transfer more heat to the panelling.
Test Your Knowledge!
Lesson 4
Outcomes:
- Explain the function of oxygen within the combustion process.
Oxygen
Oxygen in the air is the primary oxidizing agent in most fires. Normally, air consists of about 21 percent oxygen. The energy release in fire is directly proportional to the amount of oxygen available for combustion. When a fire ignites in an open area where air is plentiful, the fire will release energy based on the given surface area.
In contrast, when a fire ignites within a compartment with limited air supply the fire can only react with oxygen from the compartment’s air and any additional oxygen supplied through openings. Thus, in most compartment fires, the energy released is proportional to the limited amount of oxygen available, not the amount of fuel available to burn.
Combustion at Varied Oxygen Levels
- At normal ambient temperatures (68°F [20°C]), materials can ignite and burn at oxygen concentrations as low as 15 percent.
- At high ambient temperatures, flaming combustion may continue at considerably lower oxygen concentrations.
- When oxygen concentration is limited, the flaming combustion will diminish, causing combustion to continue smouldering (non-flaming combustion).
- Non-flaming or smouldering combustion can continue at extremely low oxygen concentrations even when the surrounding environment’s temperature is relatively low.
Oxygen-enriched atmospheres
- Oxygen-enriched atmospheres cause materials to ignite more easily and burn more intensely; certain materials, like Nomex® fire-resistant fabric, only burn in these enriched conditions.
- Nomex® is used in many types of protective clothing. At normal oxygen levels, Nomex® does not burn. When placed in an oxygen-enriched atmosphere of approximately 31 percent oxygen, Nomex® ignites and burns vigorously.
- Some petroleum-based materials will autoignite in oxygen-enriched atmospheres.
- Fires in oxygen-enriched atmospheres are more difficult to extinguish and present a potential safety hazard.
- Firefighters may find these conditions in hospitals and other healthcare facilities, some industrial occupancies, and even private homes where occupants use breathing equipment containing pure oxygen.
For combustion to occur after a fuel converts into a gaseous state, the fuel must be mixed with air (an oxidizer) in the proper ratio.
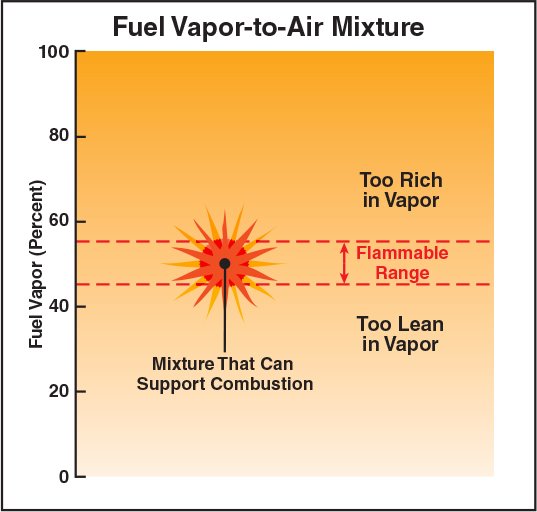
Flammable (explosive) Range: The range of concentrations of the fuel vapour and air.
The fuel’s flammable range is reported using the percent by volume of gas or vapour in air for the lower explosive (flammable) limit (LEL) and for the upper explosive (flammable) limit (UEL).
Lower explosive (flammable) limit (LEL): The minimum concentration of fuel vapour and air that supports combustion. Concentrations below the LEL are said to be too lean to burn.
Upper explosive (flammable) limit (UEL): The concentration above which combustion cannot take place. Concentrations above the UEL are said to be too rich to burn.
The flammable range is a relatively narrow band of conditions at which a mixture of fuel vapors and air will burn. Within the flammable range, there is an ideal concentration at which there is exactly the correct amount of fuel and oxygen required for combustion (Figure 4.27).
Test Your Knowledge!
Lesson 5
Outcomes:
- Explain the self-sustained chemical reaction involved in flaming combustion.
Self-Sustained Chemical Reaction
The self-sustained chemical reaction involved in flaming combustion is complex. As flaming combustion occurs, the molecules of a fuel gas and oxygen (O2 parts of molecules) break apart to form free radicals (electrically charged, highly reactive parts of molecules). Free radicals combine with oxygen or with the elements released from the fuel gas to form new substances (molecules) and even more free radicals. The process also increases the speed of the oxidation reaction.
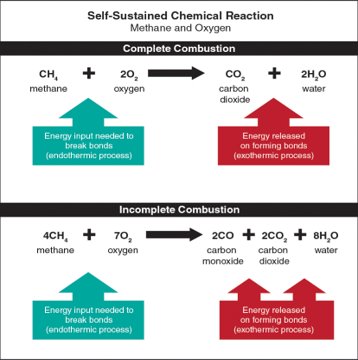
For example, let’s look at the combustion of a simple fuel such as methane and oxygen:
- Methane reacts with oxygen, initiating combustion.
- The carbon and hydrogen released from methane as its molecules break down, recombine with oxygen in the air to form carbon dioxide (CO2) and water (H2O) (Figure 4.28).
- At various points during combustion, intermediate products such as carbon monoxide (CO) and formaldehyde form, which are both flammable and toxic.
- Complete oxidation of methane releases carbon, hydrogen, heat, and light.
When more chemically complex fuels burn, their combustion creates different types of free radicals and intermediate combustion products, many of which are also flammable and toxic.
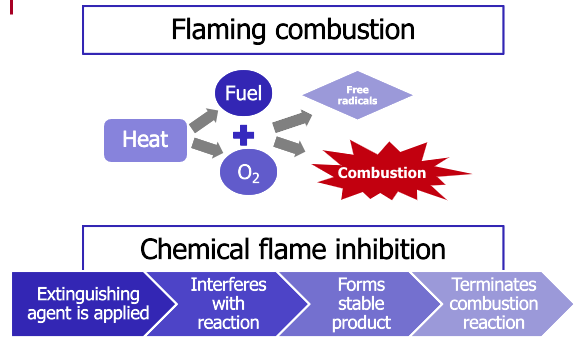
Flaming combustion is one example of a chemical chain reaction. Sufficient heat will cause fuel and oxygen to form free radicals and initiate the self-sustained chemical reaction. Fires like this will continue to burn until they consume all the fuel or oxygen or an extinguishing agent, applied in sufficient quantity, interferes with the ongoing reaction.
Chemical flame inhibition occurs when an extinguishing agent, such as a dry chemical or Halon-replacement agent, interferes with this chemical reaction, forms a stable product, and terminates the combustion reaction.
Lesson 6
Outcomes:
- Differentiate among the stages of fire development.
Compartment Fire Development
Typically, when we think about a fire, we tend to limit our perspective to the burning fuel itself. However, in the sections that follow, we will see that the compartment surrounding that burning fuel has a significant impact on the available ventilation, access to additional fuel, and heat losses or gains.
Compartment fire development depends upon whether the fire is:
- Fuel-limited
When sufficient oxygen is available for flaming combustion. Under these conditions, the fuel’s characteristics such as heat release rate and configuration control fire development. As long as the fire can reach more ignitable fuel, it will continue to burn. - Ventilation-limited
The fire has access to all of the fuel needed to maintain combustion. However, the fire does not have access to enough oxygen to continue to burn or to spread to all available fuels.
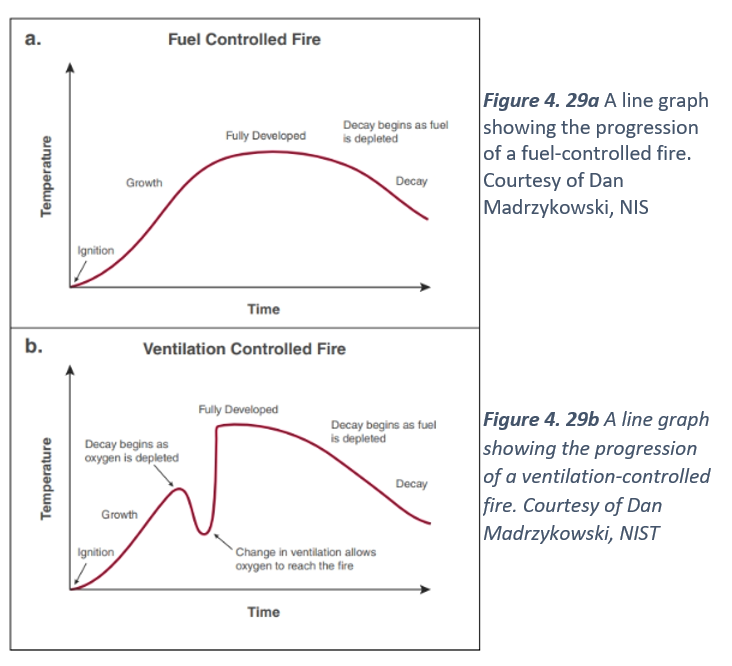
All compartment fires begin in the incipient stage as fuel-limited fires. Once the fire reaches the growth stage, the fire will either remain fuel-limited, if there is enough oxygen to support continued growth, or the fire will consume all available oxygen and become ventilation-limited. A fuel-limited fire will usually progress through the stages of fire development in order. Ventilation-limited fires tend to enter an early state of decay at the end of the growth stage because there is no longer enough available oxygen for the fire to become fully developed.
The following section will define the stages of fire development and then describe the progression of a fire in a compartment.
**NOTE: The following section’s examples in the information boxes describe fire behaviour in a room with one exterior window, an exterior doorway, and typical modern furnishings found in a residential living room.**
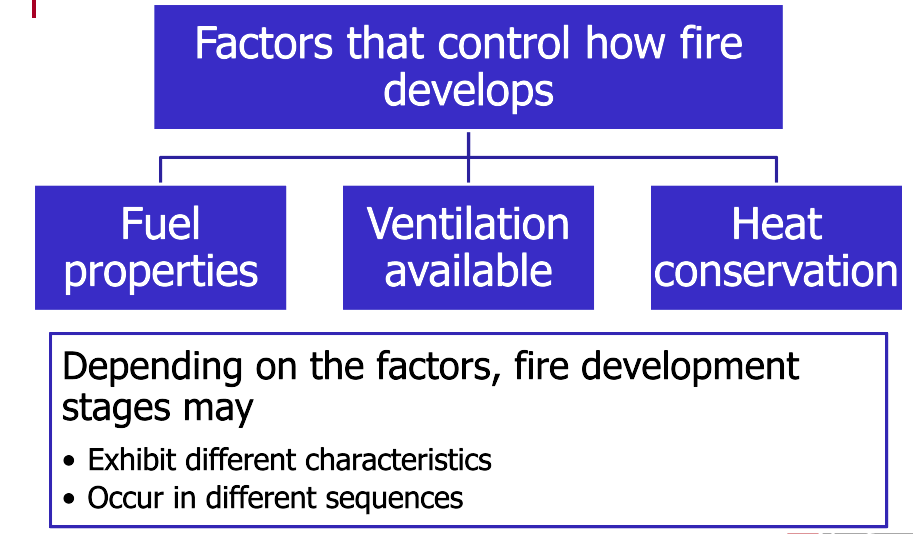
Stages of Fire Development
Fires develop through four stages: incipient, growth, fully developed, and decay. These stages can occur with any fire; however, three key factors control how the fire develops: the fuel properties, the ventilation available, and heat conservation. Depending on these factors, the fire development stages exhibit different characteristics or may occur in a different sequence.
The four stages of fire development can be generally defined as follows:
Incipient Stage
- The incipient stage starts with ignition when the three elements of the fire triangle come together and the combustion process begins.
- At this point, the fire is small and confined to a small portion of the fuel first ignited.
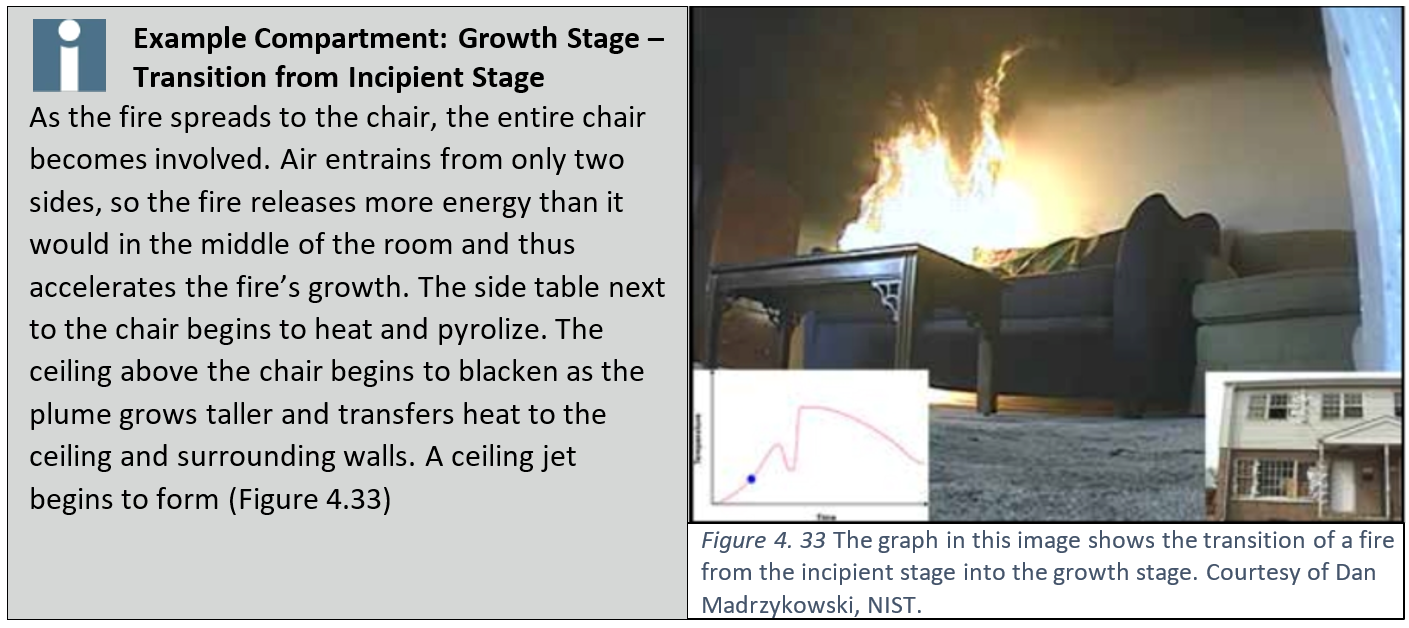
Growth Stage
- As the fire transitions from incipient to growth stage, more of the initial fuel package becomes involved and the production of heat and smoke increases.
- If there are other fuels close to the initial fuel package, radiant heat from the fire may begin to pyrolyze nearby fuels which could spread the fire to new fuel packages.
- The fire may continue to grow to become fully developed or may enter an early state of decay depending upon available oxygen.
Fully Developed Stage
- The fully developed stage occurs when all combustible materials in the compartment are burning at their peak heat release rate based on the available oxygen.
- The fire is consuming the maximum amount of oxygen that it can.
- If the fire is limited to one fuel package, the fully developed stage occurs when the entire fuel package is on fire and the fire has reached its peak heat release rate.
Decay Stage
- As the fire consumes the available fuel or oxygen and the heat release rate begins to decline, the fire enters the decay stage.
- Fuel-limited fires may self-extinguish in this phase or reduce to smouldering fires.
- Ventilation-limited fires may also self-extinguish. However, if oxygen becomes available during the decay stage before complete extinguishment, these fires are likely to reenter the growth stage and rapidly become fully developed.
A line graph showing the progression of a fuel-controlled fire. Courtesy of Dan Madrzykowski, NISFigure 4. 29b A line graph showing the progression of a ventilation-controlled fire. Courtesy of Dan Madrzykowski, NIST
Open Burning
Open burning or a free burn condition provides the most basic fire growth curve (Figure 4.29 a and b). Open burning is representative of a fuel-limited fire, such as a campfire, a pile of wood pallets, or a sofa in a large, open, empty warehouse. This fire is considered fuel-controlled because a single item burning either outside or in a large, well-ventilated space means there is sufficient oxygen available to burn the fuel until it can no longer sustain combustion. As heat and fire gases are produced, they move away from the fuel and disperse throughout the environment remote from the burning fuel. The only limit or control on the heat release rate of a fire burning out in the open is the fuel itself.
Incipient Stage
The incipient stage is where a fire begins (Figure 4.30). Once ignition occurs and the combustion process begins, development in the incipient stage depends largely upon the characteristics and configuration of the fuel involved (fuel-limited fire). Air in the compartment provides adequate oxygen to continue fire development. The following describes what occurs when a compartment fire enters the incipient stage:
- Radiant heat warms the adjacent fuel and continues the process of pyrolysis. A thin plume of hot gases and flame rises from the fire and mixes with the cooler air in the compartment.
- The hot gases in the plume rise until they encounter the ceiling and then begin to spread horizontally. This flow of fire gases is called the ceiling jet.
- Hot gases in contact with the surfaces of the compartment and its contents transfer heat to other materials.

Figure 4. 30 An example of an incipient fire on a couch. Courtesy of Dan Madrzykowski, NIST
In this early stage of fire development, the fire has not yet influenced the environment within the compartment to a significant extent. The temperature, while increasing, is only slightly above ambient in areas that the fire, plume, and ceiling jet directly affect. During the incipient stage, occupants can safely escape from the compartment, and a portable extinguisher or small hoseline can safely extinguish the fire.
- Fire development depends on characteristics and configuration of fuel involved
- Radiant heat warms adjacent fuel and pyrolysis continues
- Thin plume of hot gases and flame rise from the fire and spread across the ceiling (ceiling jet)
- Hot gases transfer heat to other materials
- Fire has not significantly influenced the environment

The transition from incipient to growth stage can occur quickly (in some cases in seconds), depending on the type and configuration of fuel involved. A visual indicator that a fire is leaving the incipient stage is flame height. When flames reach 2.5 feet (750 mm) high, radiated heat begins to transfer more heat than convection. The fire will then enter the growth stage.

Growth Stage
Within the growth stage, a variety of fire behaviours can occur, depending upon the number of ventilation sources. The fire may consume all of its available oxygen and enter a ventilation-limited state of decay or ventilation may provide enough oxygen for rapid fire development and/or growth to full development. Rapid fire development usually occurs during the growth stage. Understanding fire dynamics is largely an understanding of everything that can happen during the growth stage.
**NOTE: Keep in mind that if the fire enters ventilation-limited decay, it does not necessarily indicate that the fire is in its final stage of development. **
Incipient -> Growth Stage
- As the fire transitions from incipient to growth stage, it begins to influence more of the compartment’s environment.
- It has grown large enough for the compartment configuration and amount of ventilation to influence it.
- The first effect is the amount of air that is entrained into the fire.
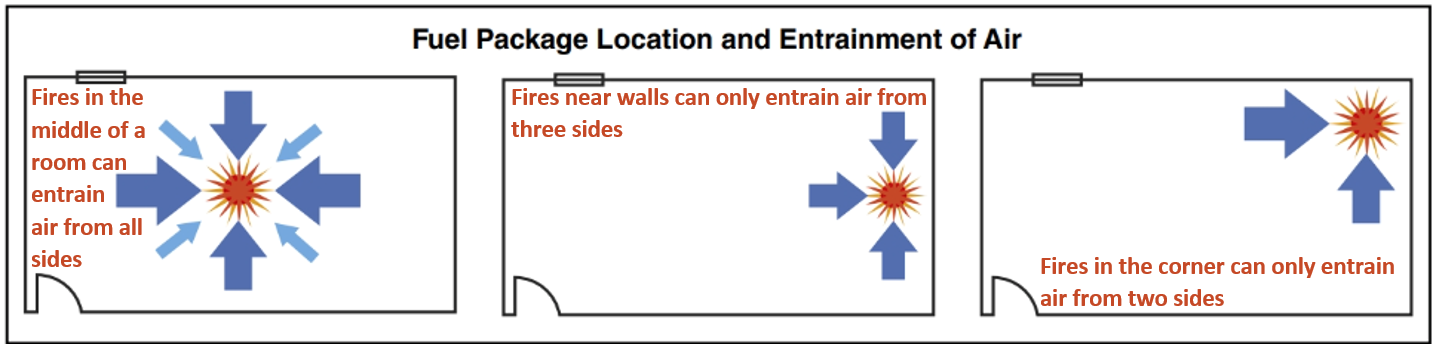
Entrainment
- Unconfined fires draw air from all sides and the entrainment (drawing in) of air cools the plume of hot gases reducing flame length and vertical extension (Figure 4.32).
- In a compartment fire, the location of the fuel package in relation to the compartment walls affects the amount of air that is entrained and thus the amount of cooling that takes place.
- The following tenets describe entrainment based on the positioning of fuel packages:
- Fires in fuel packages in the middle of the room can entrain air from all sides.
- Fires in fuel packages near walls can only entrain air from three sides.
- Fires in fuel packages in corners can only entrain air from two sides.
- When the fuel package is not in the middle of the room, the combustion zone expands vertically.
Combustion Zone
- When the fuel package is not in the middle of the room, the combustion zone expands vertically and a higher plume results.
- A higher plume increases the temperatures in the developing hot gas layer at ceiling level and increases the speed of fire development.
- The heated surfaces around the fire radiate heat back toward the burning fuel which further increases the speed of fire development.
A fire is in the growth stage until the fire’s heat release rate has reached its peak, either because of a lack of fuel or a lack of oxygen. In other words, when a fire cannot grow without the introduction of a new fuel source or a new oxygen source, it has left the growth stage and become fully developed.
Two common routes to full development are:
- Fires that consume all available oxygen and transition to a state of ventilation-limited decay.
- Fires that have enough oxygen and move through the growth phase and possibly into rapid fire development.
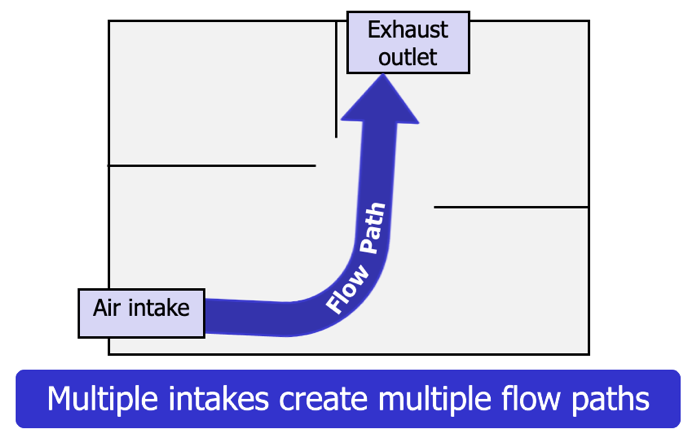
Thermal Layering
Thermal layering: The tendency of gases to form into layers according to temperature, gas density, and pressure.
Flow path: The space between the air intake and the exhaust outlet. Multiple openings (intakes and exhausts) create multiple flow paths.

Once the ceiling jet reaches the walls of the fire compartment, the hot gas layer begins to develop. Provided that there is no mechanical mixing from a fan or a hose stream, the hottest gases will form the highest layer, while the cooler gases will form the lower layers (Figure 4.34).In addition to the effects of heat transfer through radiation and convection described earlier, radiation from the hot gas layer also acts to heat the interior surfaces of the compartment and its contents. Changes in ventilation and flow path can significantly alter thermal layering.
Products of Combustion
- The products of combustion from the fire begin to affect the environment within the compartment.
- As the fire continues to grow, the hot gas layer within the fire compartment gains mass and energy.
- As the mass and energy of the hot gas layer increases, so does the pressure.
- Higher pressure causes the hot gas layer to spread downward within the compartment and laterally through any openings such as doors or windows.
- If there are no openings for lateral movement, the higher-pressure gases have no lateral path to follow to an area of lower pressure. As a result, the hot gases will begin to fill the compartment starting at the ceiling and filling down.
Isolated or Intermittent Flames
-

Figure 4. 35 Illustrating the location of the neutral plane in a compartment fire. Hot gases are exiting through the upper part of the doorway while cooler air enters through the lower part of the doorway. Courtesy of Dan Madrzykowski, NIST. Isolated or Intermittent Flames may move through the hot gas layer.
- The combustion of these hot gases indicates that portions of the hot gas layer are within their flammable range and that there is
- sufficient heat to cause ignition.
- As these hot gases circulate to the outer edges of the plume or the lower edges of the hot gas layer, they find sufficient oxygen to ignite.
- This phenomenon frequently occurs before more substantial involvement of flammable products of combustion in the hot gas layer.
- The appearance of isolated flames is sometimes an immediate indicator of flashover.
Neutral Plane
Neutral Plane: The interface between the hot gas layers and the cooler layer of air, because the net pressure is zero, or neutral, where the layers meet.
- The neutral plane exists at openings where hot gases exit and cooler air enters the compartment. At these openings, hot gases at higher than ambient pressure exit through the top of the opening above the neutral plane. Lower-pressure air from outside the compartment entrains into the opening below the neutral plane (Figure 4.35).

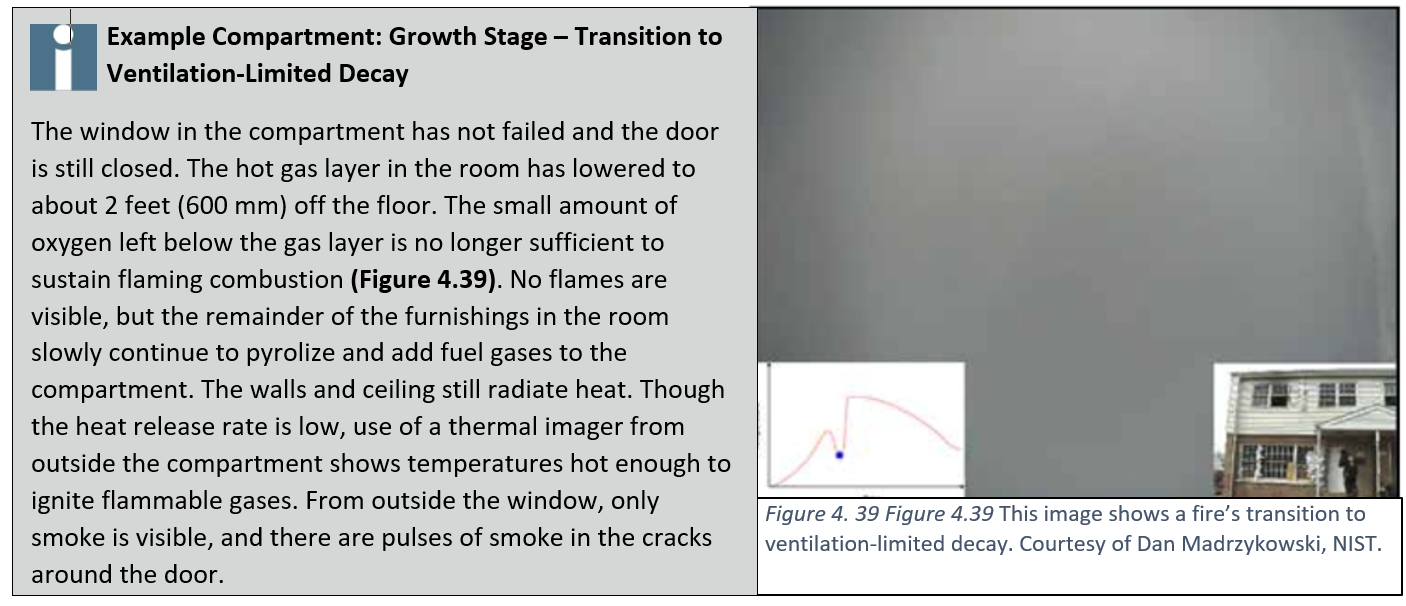
Transition to Ventilation-Limited Decay
Most residential fires that develop beyond the incipient stage become ventilation-limited. Even when doors and windows are open, insufficient air entrainment may prohibit the fire from developing based on the available fuel. When windows are intact and doors are closed, the fire may move into a ventilation-limited state of decay even more quickly. While a closed compartment reduces the heat release rate, fuel may continue to pyrolyze, creating fuel-rich smoke.
Interrupting the Entrainment
As the interface height of the hot gas layer descends toward the floor, the greater volume of smoke begins to interrupt the entrainment of fresh air and oxygen to the seat of the fire and into the plume. This interruption causes the fire within the compartment to burn less efficiently. As the efficiency of combustion decreases (incomplete combustion), the heat release rate decreases and the amount of unburned fuel within the hot gas layer increases.
The fire is now in a state of ventilation-limited decay because:
- There is not enough oxygen to maintain combustion
- The heat release rate has decreased to the point that fuel gases will not ignite.
Although the heat release rate decreases when a fire is ventilation-limited, the temperature in the room may remain high. Because there is not enough oxygen to maintain combustion, the fire has a lower heat release rate, but that does not mean that the environment is tenable. The compartment fills with fuel-rich gases that only need more oxygen to ignite because of the higher temperatures in the compartment.
Even if temperatures decrease, pyrolysis can continue. Under these conditions, a large volume of flammable products of combustion can accumulate within the compartment. These gases are fuel that can ignite when given a new source of oxygen.
If no other source of oxygen exists, the compartment will fill with black smoke and slowly cool fuel gases. The compartment will show no visible flames. The characteristics of the fuel and fuel load in today’s typical fires will cause fires to quickly become ventilation-limited.

Ventilation-limited Fire Growth
- For a ventilation-limited fire to grow, it needs a new supply of oxygen.
- Ventilation introduces outside air to the fire as this new source of oxygen.
- If windows or doors fail, the sudden introduction of fresh air creates a rapid increase in the heat release rate and growth of the fire.
- This rapid increase can also occur when firefighters open a door or window to enter the compartment for extinguishment, which creates a new flow path (Figure 4.37).
![]()
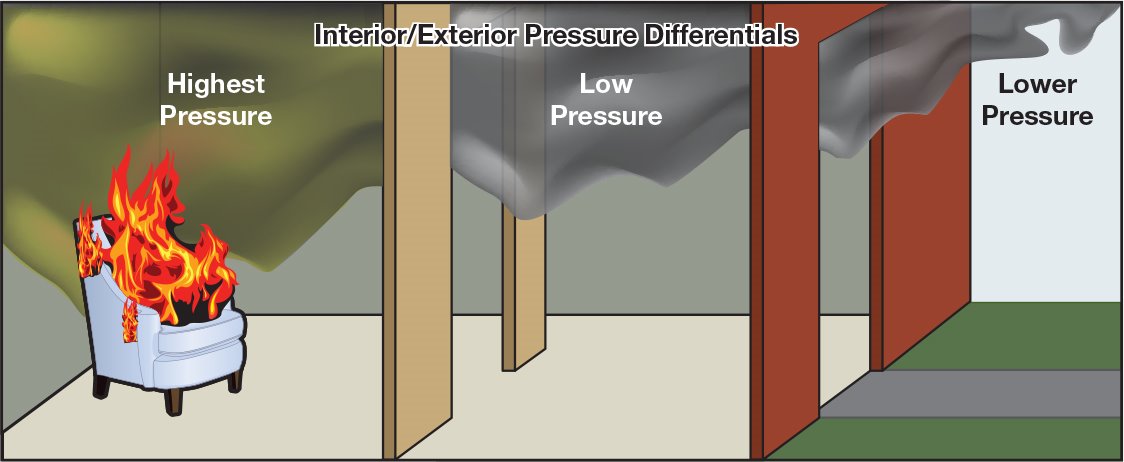
Pressure Differences
The pressure outside the compartment is lower than the pressure inside the compartment (Figure 4.38). Because of these pressure differences, any ventilation to the outside – opening an interior or exterior door, or breaking or opening a window – provides a flow path along which the hot gases can now move from the high-pressure area inside to the low-pressure area outside.
Rapid Fire Development
Rapid fire development refers to the rapid transition from the growth stage or early decay stage to a ventilation-limited, fully developed stage (Figure 4.40). Among these events are flashover and backdraft.
**NOTE: Smoke explosions are also incidents of rapid fire development, but they involve more than just one compartment of a structure. Smoke explosions will be described later in this chapter **
Rapid fire development has been responsible for numerous firefighter deaths and injuries. To protect yourself and your crew, you must be able to:
- Recognize the indicators of rapid fire development
- Know the conditions created by each of these situations
- Determine the best action to take before they occur
Flashover
Flashover is the Rapid transition from the growth stage to the fully developed stage.
- When flashover occurs, the combustible materials and fuel gases in the compartment ignite almost simultaneously; the result is full-room fire involvement.
- Flashover typically occurs during the fire’s growth stage but may occur during the fully developed stage as the result of a change in ventilation.
- Flashover conditions are defined in various ways; however, during flashover, the environment of the room changes from a two-layer condition (hot on top, cooler on the bottom) to a single, well–mixed hot gas condition from floor to ceiling. The environment is untenable, even for fully protected firefighters.
- As flashover occurs, the gas temperatures in the room reach 1,100 °F (593°C) or higher.